Public Companies: NewLink Genetics

JOE GARDYASZ Jan 16, 2015 | 12:00 pm
5 min read time
1,201 wordsBusiness Record Insider, Health and WellnessHeadquarters: Ames
Website: www.newlinkgenetics.com
Ticker symbol: NLNK
Employees: 120
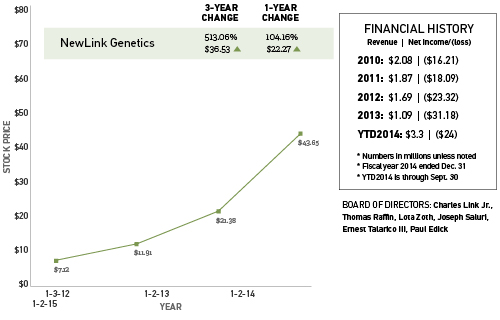
NewLink Genetics Corp. has grabbed national headlines recently for its work on a possible vaccine to turn the tide of the West African Ebola epidemic. However, the Ames-based biopharmaceutical company’s core products and financial future remain firmly ensconced in several promising types of cancer vaccines.
For the past 15 years, NewLink Genetics has been developing immunotherapies that unleash the body’s innate abilities to fight cancer.
The company was launched in 1999 by Dr. Charles Link and Dr. Nick Vahanian, both of whom believe that immunotherapy is the future of cancer treatment. Early work in this area was focused on overcoming either the failure of the immune system to recognize cancer, or the ability of the tumor to suppress the immune system. However, NewLink Genetics foresaw that the true potential of cancer immunotherapy would come from addressing both of these immune system deficits.
NewLink’s HyperAcute immunotherapy platform creates novel biologic products that are designed to stimulate the human immune system to recognize and attack cancer cells. NewLink’s lead product candidate in this class, called algenpantucel-L (HyperAcute pancreas), is being studied in a phase three drug trial with 722 patients being treated for pancreatic cancer. Additional versions of the HyperAcute immunotherapies are in development for other cancers, including lung, prostate and renal cancers and melanoma.
One of the company’s most significant breakthroughs came in October 2014, when NewLink entered into a exclusive worldwide license agreement with drugmaker Genentech for the development of NLG919, a type of cancer-fighting compound developed by NewLink known as an IDO pathway inhibitor. The companies also entered into a research collaboration for the discovery of next-generation compounds.
“As we announced in August, we continue to develop the science around combining our IDO pathway product candidates with treatment modalities including other checkpoint inhibitors, chemotherapy and our own HyperAcute immunotherapies,” Link said in November in its third-quarter report. The license and collaboration agreement with Genentech “is the most important recent step in realizing that vision, in addition to providing us with substantial new capital to develop further our HyperAcute vaccine technology.”
Under the terms of the agreement with Genentech, NewLink will receive an upfront payment of $150 million. NewLink will be eligible to receive in excess of $1 billion in milestone payments based on achievement of certain predetermined milestones as well as escalating double-digit royalties on potential commercial sales of multiple products by Genentech.
NewLink is also working to independently develop another IDO pathway inhibitor called Indoximod, which is being tested for breast cancer and prostate cancer.
The company issued its initial public offering of stock in November 2011. In the most recent financial statement, NewLink reported a net loss of $5.6 million for the third quarter of 2014, compared with a net loss of $8.1 million for the comparable period in 2013.
Looking back, what kind of a year was 2014?
It’s been essentially our best commercial development year in the history of the company. We have been working on a project over the last 10 years for a new class of drugs, which as a strategy is turning out to be very successful. We’re working with a class of molecules called checkpoint inhibitors. … These types of drugs may work in a dozen or more types of cancer, so it’s changing the dynamics of the cancer market. That’s attractive because it means the work may be able to be leveraged on several types of cancer.
Q & A with Charles Link, ceo, NewLink Genetics
Age: 55
Education: Bachelor of Arts, Stanford University; Doctor of Medicine, Stanford University Medical School; internal medicine training, University of California, San Francisco; fellowship training in medical oncology, National Cancer Institute
Hometown: Bellaire, Ohio
Family: Married with three children
How did the Genentech deal come about?
The Genentech deal was the end result of a competitive process that began with several companies, chosen, in part, on which we thought were most scientifically aligned with our processes. We wanted a company that was enthusiastic about the role of checkpoint inhibitors. That’s always been a central theme to NewLink – that immuno-oncology was going to be a combination of approaches. … There are T-cells that attach to and kill cancer cells. Each T-cell has a specific signature protein it wants to attack. Before you (administer) a checkpoint inhibitor, half of the patients may not have any measurable T-cells for that cancer. The theory, and we believe there’s a lot of proof surrounding it, is that if you give a cancer vaccine and stimulate artificially T-cells against that cancer type, you can change the T-cell population to have an anti-cancer signature, then checkpoint inhibitors allow them to expand and be clinically successful.
Could the HyperAcute immunotherapy vaccines be the first to get to market?
We’ve been working on this product for almost 15 years and have created a lot of intellectual property around it. It’s enrolled in trials in 75 universities and top cancer centers across the United States. An interim analysis is expected in the first quarter of 2015, with full results by the end of the year. This has received pre-negotiated approval from the U.S. Food and Drug Administration, so if it meets the milestones, it would potentially be our first product in the U.S. market. Our intent is to market this ourselves, without a big corporate collaborator. There are six different vaccines; the pancreatic cancer vaccine is the most advanced.
What’s ahead in the next five years?
Our goal is to be a fully integrated biopharmaceutical company. We have to build a commercialization structure to bring our products to market. We have hired a new chief financial officer who is a very experienced commercial person. With that, we also have to build a commercial sales team to work with the federal government. We’re basing the commercial team out of Austin, Texas. The pharmaceutical development groups will all remain and are expanding in Iowa. Our research and development expense this past year was about $9 million. We anticipate we’ll have over $200 million in (capital) in the company in January. So we’re in tremendous financial shape.
What about the infectious disease arm of NewLink?
We have been working on (infectious disease vaccines) for almost seven years, and we turned out to have one of two Ebola vaccine products that are furthest along. We have already completed manufacture of 2 million doses, and we have a total of seven phase one trials underway. Our plan is to launch large-scale efficacy trials in January in Africa. It’s happening at ridiculously fast speed; I’ve never seen anything like it to move an experimental drug forward so fast. … Everybody wants to help; it’s a project that’s really self-funding at this point. It’s really an extraordinary undertaking.
How might employment scale up?
Right now we have a total of 120 employees, and we have about 20 positions open. In three to five years, I anticipate we will have 500 to 800 employees. Probably 20 to 30 percent of those employees will be in Austin and the remainder in Ames. We’re already designing new buildings. We’ve got a really busy year ahead. We hope to see our hopes and dreams come to fruition.